General
 CDC and FDA Recommend Updated (2024-2025) Respiratory Virus Vaccines
Health Emergency Management - General
September 3, 2024
CDC and FDA Recommend Updated (2024-2025) Respiratory Virus Vaccines
Health Emergency Management - General
September 3, 2024
On August 22, 2024, the FDA approved and granted emergency use authorization (EUA) to the 2024-2025 formulation of both the Moderna COVID-19 and the Pfizer-BioNTech COVID-19 vaccines for everyone 6 months of age and older. On August 30, the FDA granted EUA to the updated 2024-2025Novavax COVID-19 vaccine. The updated COVID vaccines were also endorsed by the Advisory Committee on Immunization Practices (ACIP) and the CDC.
 Vaccine Purchase Act Provider Listing 2025
Immunization - General
September 2, 2024
Vaccine Purchase Act Provider Listing 2025
Immunization - General
September 2, 2024
This is a list of provider names.
 Vaccine Purchase Act Cost Estimates FY25
Immunization - General
August 30, 2024
Vaccine Purchase Act Cost Estimates FY25
Immunization - General
August 30, 2024
This is a list of provider cost list
 CD Manual - Acute Flaccid Myelitis
Infectious Disease Surveillance - General
August 28, 2024
CD Manual - Acute Flaccid Myelitis
Infectious Disease Surveillance - General
August 28, 2024
Acute Flaccid Myelitis
 CD Manual - Meningococcal Disease
Infectious Disease Surveillance - General
August 26, 2024
CD Manual - Meningococcal Disease
Infectious Disease Surveillance - General
August 26, 2024
Meningococcal Disease
Meningococcal Factsheet (English)
Infeccion Meningococica Factsheet (Spanish)
 CD Manual - Measles (Rubeola)
Infectious Disease Surveillance - General
August 26, 2024
CD Manual - Measles (Rubeola)
Infectious Disease Surveillance - General
August 26, 2024
Measles (Rubeola)
 Environmental Protection Agency Drinking Water Certificate for Chemistry & Microbiology
Scientific Laboratory - General
August 21, 2024
Environmental Protection Agency Drinking Water Certificate for Chemistry & Microbiology
Scientific Laboratory - General
August 21, 2024
The United States Environmental Protection Agency region 6 certifies that the New Mexico Department of Health Scientific Laboratory Division has bet the requirements for certification of laboratories analyzing drinking water and is hereby certified to conduct analysis of drinking water samples for inorganic chemistry and radiochemistry as specified in the scope of certification.
 Increase in Human Parvovirus B19 Activity in the United States
Health Emergency Management - General
August 13, 2024
Increase in Human Parvovirus B19 Activity in the United States
Health Emergency Management - General
August 13, 2024
Increase in Human Parvovirus B19 Activity in the United States
 Proclamation - World Breastfeeding Month 2024
Family Health - General
July 25, 2024
Proclamation - World Breastfeeding Month 2024
Family Health - General
July 25, 2024
Proclamation - World Breastfeeding Week 2024
 NMHSC Stipend Reference Letter for 2024 - 2025
Primary Care & Rural Health - General
July 9, 2024
NMHSC Stipend Reference Letter for 2024 - 2025
Primary Care & Rural Health - General
July 9, 2024
NMHSC Stipend Reference Letter for 2024 - 2025
 NMHSC SoNM Substitute - W9 Form 2024 - 2025
Primary Care & Rural Health - General
July 9, 2024
NMHSC SoNM Substitute - W9 Form 2024 - 2025
Primary Care & Rural Health - General
July 9, 2024
SoNM Substitute - W9 Form
 Fee Schedule for Chemistry Analysis
Chemistry - General
July 9, 2024
Fee Schedule for Chemistry Analysis
Chemistry - General
July 9, 2024
Complete fee schedule for the scientific laboratory division's chemistry bureau.
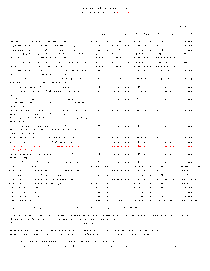 Fee Schedule for Air and Metals Analysis
Chemistry - General
July 9, 2024
Fee Schedule for Air and Metals Analysis
Chemistry - General
July 9, 2024
Complete fee schedule for the scientific laboratory division's air and heavy metals section of the chemistry bureau.
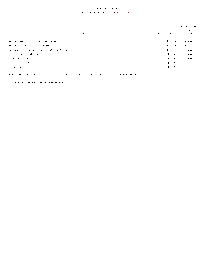 Fee Schedule for CTAR Analysis
Chemistry - General
July 9, 2024
Fee Schedule for CTAR Analysis
Chemistry - General
July 9, 2024
Complete fee schedule for the scientific laboratory division's CTAR section of the chemistry bureau.
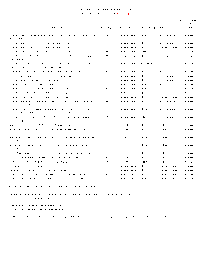 Fee Schedule for Organic Chemistry Analysis
Chemistry - General
July 9, 2024
Fee Schedule for Organic Chemistry Analysis
Chemistry - General
July 9, 2024
Complete fee schedule for the scientific laboratory division's organic chemistry section of the chemistry bureau.
 Fee Schedule for Radiochemistry Analysis
Chemistry - General
July 9, 2024
Fee Schedule for Radiochemistry Analysis
Chemistry - General
July 9, 2024
Complete fee schedule for the scientific laboratory division's radiochemistry section of the chemistry bureau.
 Fee Schedule for Water Chemistry Analysis
Chemistry - General
July 9, 2024
Fee Schedule for Water Chemistry Analysis
Chemistry - General
July 9, 2024
Complete fee schedule for the scientific laboratory division's water chemistry section of the chemistry bureau.
 Fee Schedule for Safe Drinking Water Act Analysis
Chemistry - General
July 9, 2024
Fee Schedule for Safe Drinking Water Act Analysis
Chemistry - General
July 9, 2024
Complete fee schedule for the scientific laboratory division's safe drinking water section of the chemistry bureau.




