General
 Plan-Do-Study-Act
Quality Management - General
January 22, 2020
Plan-Do-Study-Act
Quality Management - General
January 22, 2020
PDSA, or Plan-Do-Study-Act, is an iterative, four-stage problem-solving model used for improving a process or carrying out change.
 Run Chart
Quality Management - General
January 22, 2020
Run Chart
Quality Management - General
January 22, 2020
A run chart is used to study collected data for trends or patterns over a specific period of time.
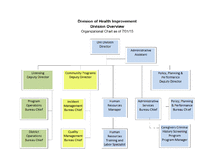 Division of Health Improvement Organizational Structure
Human Resources - General
January 22, 2020
Division of Health Improvement Organizational Structure
Human Resources - General
January 22, 2020
This document provides the latest organizational structure for the Division of Health Improvement.
 Clinical Laboratory Improvement Act Historical Information
Program Operations - General
January 22, 2020
Clinical Laboratory Improvement Act Historical Information
Program Operations - General
January 22, 2020
The Clinical Laboratory Improvement Act began in the late 1960's when problems arose in the cytology laboratories that read PAP smears. The personnel in these laboratories were overworked and had a very high error rate. In 1967, the Clinical Laboratory Improvement Amendment was passed and the first laboratory regulations were born. These regulations primarily covered independent and hospital laboratories.
 Live Blood Cell Analysis Under the Clinical Laboratory Improvement Act
Program Operations - General
January 22, 2020
Live Blood Cell Analysis Under the Clinical Laboratory Improvement Act
Program Operations - General
January 22, 2020
Live Blood Cell Analysis is a test which is used for the purpose of providing information for the diagnosis, prevention, or treatment of any disease or impairment of, or assessment of the health of human beings. The Health Care Financing Administration (now the Centers for Medicare & Medicaid Services) Office of General Counsel determined in August, 1997, that Live Blood Cell Analysis was subject to all Clinical Laboratory Improvement Act requirements.
 Nurse Aide Registry Test Fee Rates
Program Operations - General
January 22, 2020
Nurse Aide Registry Test Fee Rates
Program Operations - General
January 22, 2020
Nurse Aide Registry Test Fee Rates
 College of American Pathologists Certificate
Scientific Laboratory - General
January 9, 2020
College of American Pathologists Certificate
Scientific Laboratory - General
January 9, 2020
The college of American pathologists certifies that the New Mexico Department of Health Scientific Laboratory Division has bet all applicable standards for accreditation and is hereby accredited by the college of American pathologists laboratory accreditation program.
 Eligible Provider Syndromic Surveillance Onboarding Memo
Syndromic Surveillance Reporting - General
January 8, 2020
Eligible Provider Syndromic Surveillance Onboarding Memo
Syndromic Surveillance Reporting - General
January 8, 2020
Eligible Provider Syndromic Surveillance Onboarding Memo
 EMS Disciplinary Actions
EMS Committees - General
December 27, 2019
EMS Disciplinary Actions
EMS Committees - General
December 27, 2019
The information in this section is intended to provide public notice of disciplinary actions taken by the New Mexico Department of Health and the Emergency Medical Systems Bureau. Note that there may be various actions that are still in process, but have not yet been completed due to the administrative process.
 Medical Cannabis 2019 Plant Count Increase
Medical Cannabis Licensees (Dispensaries) - General
December 26, 2019
Medical Cannabis 2019 Plant Count Increase
Medical Cannabis Licensees (Dispensaries) - General
December 26, 2019
Medical Cannabis 2019 Plant Count Increase
 American Board of Forensic Toxicology Certificate
Scientific Laboratory - General
December 18, 2019
American Board of Forensic Toxicology Certificate
Scientific Laboratory - General
December 18, 2019
The American board of forensic toxicology hereby declares that this board’s standards for laboratory accreditation have been fulfilled and the other requirements of this board have been met and therefore grants this certificate of laboratory accreditation in forensic toxicology to the New Mexico Department of Health Scientific Laboratory Division’s toxicology bureau.
 Vaccine Purchase Act Cost Estimates FY21
Immunization - General
November 27, 2019
Vaccine Purchase Act Cost Estimates FY21
Immunization - General
November 27, 2019
This document provides the cost estimate of purchasing vaccine for privately insured children in New Mexico.
 NMSIIS Data Exchange Locations
Immunization - General
November 22, 2019
NMSIIS Data Exchange Locations
Immunization - General
November 22, 2019
This is the list of NM Statewide Immunization Information System Data Exchange locations.
 CD Manual - Hepatitis B
Infectious Disease Surveillance - General
November 5, 2019
CD Manual - Hepatitis B
Infectious Disease Surveillance - General
November 5, 2019
Hepatitis B
Hepatitis B Factsheet (English)
Hepatitis B Factsheet (Spanish)
 Food and Drug Administration Certificate
Scientific Laboratory - General
October 11, 2019
Food and Drug Administration Certificate
Scientific Laboratory - General
October 11, 2019
The Food and Drug Administration’s laboratory proficiency and evaluation team certifies that the New Mexico Department of Health Scientific Laboratory’s milk laboratory is at full accreditation.
 List of Districts
Public Relations - General
September 25, 2019
List of Districts
Public Relations - General
September 25, 2019
This document contains the full list of districts that received funds from the New Mexico-Grown Local Produce Grant.
 Key Facts about Influenza
Influenza Surveillance - General
September 19, 2019
Key Facts about Influenza
Influenza Surveillance - General
September 19, 2019
This document covers what the flu is, prevention, symptoms, and treatment.
 Gastrointestinal (GI) Outbreaks Toolkit for Long Term Care Facilities (LTCF)
Norovirus - General
September 9, 2019
Gastrointestinal (GI) Outbreaks Toolkit for Long Term Care Facilities (LTCF)
Norovirus - General
September 9, 2019
Gastrointestinal (GI) Outbreaks Toolkit for Long Term Care Facilities (LTCF)
 Hospital Disaster Preparedness Self-Assessment Tool
Healthcare Preparedness and Response - General
August 16, 2019
Hospital Disaster Preparedness Self-Assessment Tool
Healthcare Preparedness and Response - General
August 16, 2019
This assessment tool was developed to assist hospitals in revising and updating existing disaster plans or in the development of new plans.
 ASPR TRACIE Evaluation of Hazard Vulnerability Assessment Tools
Healthcare Preparedness and Response - General
August 16, 2019
ASPR TRACIE Evaluation of Hazard Vulnerability Assessment Tools
Healthcare Preparedness and Response - General
August 16, 2019
The ASPR TRACIE Evaluation of Hazard Vulnerability Assessment Tools provides a comparison chart showing the similarities and differences among several of the primary and other sample hazard vulnerability tools used by public health and healthcare organizations, and the Federal Emergency Management Agency’s (FEMA) Threat and Hazard Identification Risk Assessment (THIRA).


