Immunization Program
Publications
 Health Care Facility Infection Control Measures for Suspect Measles Patients
Immunization - General
June 4, 2019
Health Care Facility Infection Control Measures for Suspect Measles Patients
Immunization - General
June 4, 2019
Suspect measles in patients with febrile rash illness consistent with measle and a history in the prior 3 weeks of: contact with known or possible exposure to a measles case in the community; travel outside of North America; domestic travel via international airports; visiting US tourist attractions; or travel to areas in US with current measles transmission.
 Guidance for people exposed to measles
Immunization - General
June 4, 2019
Guidance for people exposed to measles
Immunization - General
June 4, 2019
This document outlines instructions for people exposed to measles
 VFC Transport Instructions for Refrigerated Vaccines
Immunization - Help
February 8, 2019
VFC Transport Instructions for Refrigerated Vaccines
Immunization - Help
February 8, 2019
Ideally, vaccine should be transported using a portable vaccine refrigerator or qualified pack-out. However, if these options are not available, you can follow the emergency packing procedures for refrigerated vaccines explained in this document.
 VFC Vaccine Administration Form (Part B) Instructions
Immunization - Help
February 8, 2019
VFC Vaccine Administration Form (Part B) Instructions
Immunization - Help
February 8, 2019
These instructions are designed to assist your practice or clinic staff with the entry of accurate client information into NMSIIS. Providers should continue to enter immunization information directly into NMSIIS. Part B forms will continue to be accepted for those sites that have been approved by the NMDOH Immunization Program for paper submissions.
 VFC Transport Instructions for Frozen Vaccines
Immunization - Help
November 28, 2018
VFC Transport Instructions for Frozen Vaccines
Immunization - Help
November 28, 2018
Frozen vaccines are very fragile. Routine transport of varicella-containing vaccines (MMRV, and varicella vaccine) is not allowed. These vaccines should be moved and transported only when absolutely necessary.
 School Consent to Enter Student Records into NMSIIS
Immunization - Form
September 17, 2018
School Consent to Enter Student Records into NMSIIS
Immunization - Form
September 17, 2018
This is a customizable form for use by schools to collect consent from parents so students' historic immunization records may be added to NMSIIS.
 National Immunization Partnership with the Academic Pediatric Association Flyer
Immunization - Marketing
September 10, 2018
National Immunization Partnership with the Academic Pediatric Association Flyer
Immunization - Marketing
September 10, 2018
This is a document with information about the nationwide quality improvement project to improve adolescent human papillomavirus immunization rates. By joining you can earn credit towards professional certifications including MC-FP credit for 1 Part IV module or 20 Part IV points and up to 20 Performance Improvement Continuing Medical Education credits from the AAFP.
 Vaccination-Community and Off-Site Outreach Protocol and Checklists
Immunization - Policies, Protocols & Procedures
August 24, 2018
Vaccination-Community and Off-Site Outreach Protocol and Checklists
Immunization - Policies, Protocols & Procedures
August 24, 2018
This is for nurses and Department of Health staff who conduct community and offsite vaccination outreach clinics.
 NMSIIS Administering Vaccines Participant Guide
Immunization - Training
July 17, 2018
NMSIIS Administering Vaccines Participant Guide
Immunization - Training
July 17, 2018
NMSIIS Administering Vaccines Participant Guide
 NMSIIS Inventory Training Participant Guide (Part 1/2)
Immunization - Training
July 17, 2018
NMSIIS Inventory Training Participant Guide (Part 1/2)
Immunization - Training
July 17, 2018
NMSIIS Inventory Training Participant Guide (Part 1/2)
 NMSIIS Inventory Training Participant Guide (Part 2/2)
Immunization - Training
July 17, 2018
NMSIIS Inventory Training Participant Guide (Part 2/2)
Immunization - Training
July 17, 2018
NMSIIS Inventory Training Participant Guide (Part 2/2)
 VFC Provider Staff Training Policy
Immunization - Guide
June 27, 2018
VFC Provider Staff Training Policy
Immunization - Guide
June 27, 2018
The Vaccines for Children program will require these types of provider staff training in 2017.
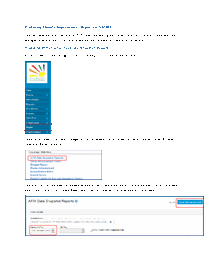 NMSIIS AFIX Data Snapshot Reports
Immunization - Help
March 23, 2018
NMSIIS AFIX Data Snapshot Reports
Immunization - Help
March 23, 2018
NMSIIS AFIX Data Snapshot Reports
 NMSIIS Immunization Rates Reports
Immunization - Help
March 23, 2018
NMSIIS Immunization Rates Reports
Immunization - Help
March 23, 2018
NMSIIS Immunization Rates Reports
 VFC Approved School Sites - Needle, Syringe, & e-kit Order Form
Immunization - Form
February 23, 2018
VFC Approved School Sites - Needle, Syringe, & e-kit Order Form
Immunization - Form
February 23, 2018
This form is to be used for needles/syringes, and e-kit medications and is for Vaccines for Children Approved Sites Only.
 VFC Middle School Vaccination Letter/Permission Form
Immunization - Form
February 23, 2018
VFC Middle School Vaccination Letter/Permission Form
Immunization - Form
February 23, 2018
This is the letter addressed to parents or legal guardians to determine whether or not a child is to be vaccinated at school. This letter is to be sent out with the VFC school immunization consent form.
VFC Middle School Vaccination Letter/Permission Form (Spanish)
 VFC Educational Requirements for Providers
Immunization - General
February 23, 2018
VFC Educational Requirements for Providers
Immunization - General
February 23, 2018
This document provides a list of the required educational requirements for the primary and backup vaccines for children contacts in your organization.
 VFC Provider Benefits
Immunization - General
February 20, 2018
VFC Provider Benefits
Immunization - General
February 20, 2018
Explains the benefits of being a Vaccines for Children provider.
 VFC Program Data Logger Timeline and Checklist
Immunization - Form
January 26, 2018
VFC Program Data Logger Timeline and Checklist
Immunization - Form
January 26, 2018
This document is for providers: guidelines and important dates for the transition to digital data loggers.
 VFC Returning Expired, Spoiled/Non-Viable or Wasted Vaccines in NMSIIS
Immunization - Help
November 15, 2017
VFC Returning Expired, Spoiled/Non-Viable or Wasted Vaccines in NMSIIS
Immunization - Help
November 15, 2017
These instructions explain how to return vaccines in the New Mexico Statewide Immunization Information System.

