Healthcare Provider Immunization Education & Tools
Vaccine Management

Proper management and storage of vaccines is critical to maintaining their biological potency. The CDC Vaccine Storage and Handling Toolkit is designed to provide guidance to immunization providers on aspects of vaccine storage and handling, transportation, equipment, standard operating procedures and training for personnel.
Adult Vaccine Ordering
Public Health Offices and in some limited circumstances Federally Qualified Health Centers may use this Adult Vaccine Order Form to order adult vaccine for use within the Adult Vaccine Screening Criteria.
Healthcare providers may use this to order adult vaccines.
If you need to return your vaccines, please use the Adult Vaccine Return Form.
Consent Forms
The Adult Vaccine Consent Form form may be used by patients aged 19 and older to provide vaccination consent to a healthcare provider.
This form is available in both English and Spanish.
Immunization Protocols

Effective vaccination requires adherence to specific protocols for each vaccine that define appropriate routes of administration, numbers of shots needed and intervals between shots, when vaccination is not recommended, and who should not receive them.
The NMDOH Immunization Protocols contains approved protocols for childhood vaccines, including DTaP, Dt, Td, Tdap, inactivated Polio, Haemophilus influenzae type b (Hib), Hepatitis B, Hepatitis A, Measles, Mumps, Rubella (MMR), Varicella, Pneumococcal conjugate vaccine, Meningococcal conjugate, Rotavirus, and HPV.
Addressing Concerns about Vaccines & Autism
Due to media exposure of two flawed medical papers, there is rampant misinformation about vaccines and autism. Parents and guardians need resources to address their questions about this topic. There is no evidence of vaccines causing autism.
Here are some resources for providers, parents, and guardians to get more information:
- Evidence Shows Vaccines Unrelated to Autism
- Vaccines and Autism: What You Should Know
- MMR Vaccine Does Not Cause Autism
- Need Help Responding to Vaccine-Hesitant Parents?
- Vaccine Resources to Share with Parents
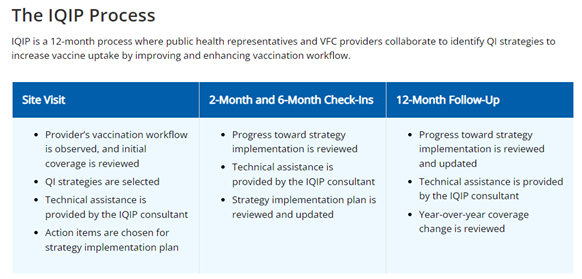
Immunization Quality Improvement for Providers
Immunization Quality Improvement for Providers (IQIP) is a CDC developed quality improvement program used by awardees to raise immunization coverage levels, reduce immunization missed opportunities, and improve standards of immunization practices at the provider level.
IQIP is a quality improvement cycle that strives to create collaboration between providers and the immunization program to improve vaccine coverage and on-time vaccination, to protect the population. This quality improvement cycle strives to cultivate collaboration to improve immunization coverage of public and private providers. Throughout the cycle feedback of immunization workflows are reviewed and improved to create a successful immunization environment, coverage levels are assessed, incentives are shared to reward improved performance of providers.
IQIP is an opportunity for providers to review their vaccine coverage levels and immunization records in the patient populations they serve to ensure on-time vaccination. It is also an opportunity to improve workflows to help increase vaccine coverage. In reviewing vaccine coverage providers can find missed opportunities to vaccine and develop strategies to close the gap to ensure on-time vaccination is occurring in all patients.
 https://www.cdc.gov/vaccines/programs/iqip/at-a-glance.html
https://www.cdc.gov/vaccines/programs/iqip/at-a-glance.html
Quality Improvement Tools for Providers in NMSIIS
VFC Providers can now produce their own NMSIIS IQIP Data Snapshot Reports and NMSIIS Immunization Rates Reports directly from NMSIIS. Data Snapshot reports provide coverage rates and lists of childhood and adolescent clients who are not up-to-date and highlight areas where missed opportunities for immunizations are occurring. Immunization Rates reports can be customized for any age group, time period, or vaccine series.
Vaccine Adverse Events Reporting System
The Vaccine Adverse Events Reporting System (VAERS) is a national vaccine safety surveillance program co-sponsored by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA).
VAERS is a post-marketing safety surveillance program, collecting information about adverse events (possible side effects) that occur after the administration of vaccines licensed for use in the United States.
VAERS provides a nationwide mechanism by which adverse events following immunization may be reported, analyzed, and made available to the public. VAERS also provides a vehicle for disseminating vaccine safety-related information to parents and guardians, health care providers, vaccine manufacturers, state vaccine programs, and other constituencies.
Resources
- Immunization Action Coalition
- Vaccine Information Statements
- New Mexico Immunization Coalition
- Vaccine Safety
- Vaccinating Adults: A Step-by-Step Guide
- Immunization of Healthcare Personnel
- Healthcare Personnel Vaccination Recommendations
- Pneumococcal polysaccharide and Conjugate Vaccines for Adults Flyer
- EZIZ
- Adult TSR Form
- COVID-19 Vaccine Troubleshooting Report

