General
 Medical Reserve Corps Packing List for Deployment
Medical Reserve Corps - General
January 22, 2011
Medical Reserve Corps Packing List for Deployment
Medical Reserve Corps - General
January 22, 2011
This document provides a helpful list of items to pack in preparation for deployment.
 EMS TSFA Authority & Bylaws
EMS Committees - General
June 8, 2010
EMS TSFA Authority & Bylaws
EMS Committees - General
June 8, 2010
This document describes the authority and bylaws for the Trauma System Fund Authority.
 EMS Air Ambulance Memo on Use of Rotor Wing Aircraft
EMS Air Abulance - General
June 8, 2010
EMS Air Ambulance Memo on Use of Rotor Wing Aircraft
EMS Air Abulance - General
June 8, 2010
This memo is a clarification of use of rotor wing aircraft during interfacility transfers.
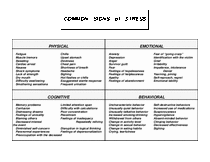 EMS Critical Incident Stress Management Common Signs of Stress
EMS Critical Incident Stress Management - General
June 8, 2010
EMS Critical Incident Stress Management Common Signs of Stress
EMS Critical Incident Stress Management - General
June 8, 2010
This document lists many common signs of stress for use in critical incident stress management.
 EMS Dispatch & Communications Narrowbanding
EMS Dispatch & Communications - General
June 8, 2010
EMS Dispatch & Communications Narrowbanding
EMS Dispatch & Communications - General
June 8, 2010
This document describes the new narrowband requirements for public safety radio equipment operating below 512 MHz which was set forth by the federal communications commission to begin on January 1, 2013.
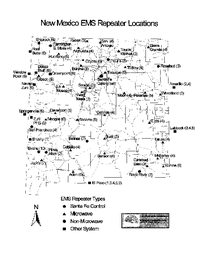 EMS Dispatch & Communications Map of Repeater Sites
EMS Dispatch & Communications - General
June 8, 2010
EMS Dispatch & Communications Map of Repeater Sites
EMS Dispatch & Communications - General
June 8, 2010
A map of emergency medical services repeater locations including Santa Fe control, microwave, non-microwave, and other system types.
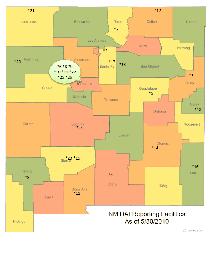 Healthcare-Associated Infections Reporting Group List of Participating Facilities
Healthcare-Associated Infections - General
May 30, 2010
Healthcare-Associated Infections Reporting Group List of Participating Facilities
Healthcare-Associated Infections - General
May 30, 2010
Map and list of facilities in New Mexico which voluntarily report Heathcare-Associated Infections.
 Center for Adolescent Relationship Exploration
Behavioral Health Institute - General
May 26, 2010
Center for Adolescent Relationship Exploration
Behavioral Health Institute - General
May 26, 2010
The Center for Adolescent Relationship Exploration program is a residential psychiatric treatment facility owned and operated by the State of New Mexico. The facility is licensed as a specialty Residential Treatment Center by the State of New Mexico and is accredited by the Joint Commission on Accreditation of Health Care Organizations.
 Cryptosporidium Investigation Flowchart Algorithm
Water Illness - General
May 20, 2010
Cryptosporidium Investigation Flowchart Algorithm
Water Illness - General
May 20, 2010
This document contains a flowchart that illustrates the steps that should be taken during a Cryptosporidium investigation.
 EMS Criminal Behavior Memo
EMS Impaired Practitioner Program - General
March 1, 2010
EMS Criminal Behavior Memo
EMS Impaired Practitioner Program - General
March 1, 2010
This memo was released for emergency medical services agency managers and providers to address criminal behavior by licensed emergency medical services caregivers.
 Water Sample Collection Protocol
Water Illness - General
May 11, 2009
Water Sample Collection Protocol
Water Illness - General
May 11, 2009
Water samples can be tested for pathogens such as Cryptosporidium. These samples can then potentially be linked to clinical isolates from individuals who visited a recreational water venue associated with cluster of cases. Collecting a sample of the backwash from a pool filter is ideal because it increases the chances of detecting the parasite if it was present during the previous filter run.
 Hyperchlorination to Kill Cryptosporidium
Water Illness - General
May 11, 2009
Hyperchlorination to Kill Cryptosporidium
Water Illness - General
May 11, 2009
Cryptosporidium (or “Crypto”) is a chlorine resistant parasite, so even well-maintained pools, water parks, and interactive fountains can spread Crypto among swimmers. If an outbreak of Crypto infections occurs in your community, the health department might ask you to hyperchlorinate.
 Cryptosporidiosis in Childcare Settings
Water Illness - General
May 11, 2009
Cryptosporidiosis in Childcare Settings
Water Illness - General
May 11, 2009
Cryptosporidiosis is a gastrointestinal illness, caused by the parasite, Cryptosporidium. This disease is a common cause of diarrhea in children, especially in child care settings. The hallmark symptom of cryptosporidiosis is watery diarrhea, which might be accompanied by stomach ache, nausea and vomiting, fever, and a general sick feeling.
 Depleted Uranium Project Summary
Biomonitoring Program - General
April 1, 2009
Depleted Uranium Project Summary
Biomonitoring Program - General
April 1, 2009
During the 2007 legislative session, Senate Bill (SB) 611 allocated $40,000 to develop a testing protocol, develop and establish a health registry, contract with appropriate testing laboratories and coordinate affected parties in regard to a voluntary testing program for military veterans who may have been exposed to depleted uranium or other isotopes in the Persian Gulf war or in the current Iraq or Afghanistan conflict.
 Federal Medical Stations Stat Sheet
Strategic National Stockpile - General
March 1, 2009
Federal Medical Stations Stat Sheet
Strategic National Stockpile - General
March 1, 2009
Significant dates, federal medical station requirements, standard features, and product information details.
 Community Environmental Health Assessment Toolbox
Environmental Health - General
October 20, 2004
Community Environmental Health Assessment Toolbox
Environmental Health - General
October 20, 2004
The Tool Box provides access to resources of varying types and specificity—from comprehensive procedural manuals and guidelines, to checklists and survey instruments, data sources, websites, and institutional contacts as well as general step-by-step guidance on best practices for implementing CEHA in New Mexican communities.
 PRAMS Survey for 2004-2008
Pregnancy Risk Assessment and Monitoring - General
January 1, 2004
PRAMS Survey for 2004-2008
Pregnancy Risk Assessment and Monitoring - General
January 1, 2004
This is the Pregnancy Risk Assessment and Monitoring System survey that was used for survey years 2004 through 2008.


