General
 SHM - Chapter 2 - Social Determinats (2016)
School and Adolescent Health - General
February 13, 2018
SHM - Chapter 2 - Social Determinats (2016)
School and Adolescent Health - General
February 13, 2018
SHM - Chapter 2 - Social Determinats (2016)
 SHM - Chapter 2 - White Paper Absenteeism
School and Adolescent Health - General
February 13, 2018
SHM - Chapter 2 - White Paper Absenteeism
School and Adolescent Health - General
February 13, 2018
SHM - Chapter 2 - White Paper Absenteeism
 SHM - Chapter 2 - Whole School, Whole Community, Whole Child Implications for 21st Century School Nurses
School and Adolescent Health - General
February 13, 2018
SHM - Chapter 2 - Whole School, Whole Community, Whole Child Implications for 21st Century School Nurses
School and Adolescent Health - General
February 13, 2018
SHM - Chapter 2 - Whole School, Whole Community, Whole Child Implications for 21st Century School Nurses
 Clinical Laboratory Improvement Amendments Certificate
Scientific Laboratory - General
February 2, 2018
Clinical Laboratory Improvement Amendments Certificate
Scientific Laboratory - General
February 2, 2018
The centers for Medicare and Medicaid services declares that the New Mexico Department of Health Scientific Laboratory Division may accept human specimens for the purposes of performing laboratory examinations or procedures.
 SHM - Chapter 3 - Audio Familiar Sounds Screen
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - Audio Familiar Sounds Screen
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - Audio Familiar Sounds Screen
 SHM - Chapter 3 - BMI Medical Oversight Position Paper
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - BMI Medical Oversight Position Paper
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - BMI Medical Oversight Position Paper
 SHM - Chapter 3 - Save Our Children’s Sight Screening Day Protocol
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - Save Our Children’s Sight Screening Day Protocol
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - Save Our Children’s Sight Screening Day Protocol
 SHM - Chapter 3 - Save Our Children’s Sight Vision Referral Letter
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - Save Our Children’s Sight Vision Referral Letter
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - Save Our Children’s Sight Vision Referral Letter
 SHM - Chapter 3 - Save Our Children’s Sight Voucher Procedure
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - Save Our Children’s Sight Voucher Procedure
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 3 - Save Our Children’s Sight Voucher Procedure
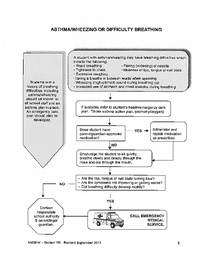 SHM - Chapter 4 - Emergency Asthma Algorithm
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 4 - Emergency Asthma Algorithm
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 4 - Emergency Asthma Algorithm
 SHM - Chapter 4 - Nursing Delegation
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 4 - Nursing Delegation
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 4 - Nursing Delegation
 SHM - Chapter 4 - Peak Flow Meter
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 4 - Peak Flow Meter
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 4 - Peak Flow Meter
 SHM - Chapter 6 - Guidelines for Hypoglycemia
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 6 - Guidelines for Hypoglycemia
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 6 - Guidelines for Hypoglycemia
 SHM - Chapter 9 - Public Health Authority Letter (2017)
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 9 - Public Health Authority Letter (2017)
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 9 - Public Health Authority Letter (2017)
 SHM - Chapter 15 - Standing Orders Signature Page (2017)
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 15 - Standing Orders Signature Page (2017)
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 15 - Standing Orders Signature Page (2017)
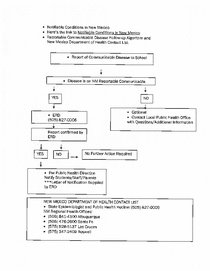 SHM - Chapter 10 - Communicable Disease Flowchart
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Communicable Disease Flowchart
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Communicable Disease Flowchart
 SHM - Chapter 10 - Notifiable Conditions Letter
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Notifiable Conditions Letter
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Notifiable Conditions Letter
 SHM - Chapter 10 - Lice Identification Photos
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Lice Identification Photos
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Lice Identification Photos
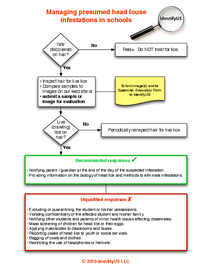 SHM - Chapter 10 - Lice Management Flowchart
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Lice Management Flowchart
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Lice Management Flowchart
 SHM - Chapter 10 - Tuberculosis Screening Guidelines
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Tuberculosis Screening Guidelines
School and Adolescent Health - General
January 25, 2018
SHM - Chapter 10 - Tuberculosis Screening Guidelines


